The visible spectrum isn't terribly big--only 390 to 700 nm--considering just how wide the electromagnetic spectrum is. Yet within that short range, we've been able to accomplish some magnificent astronomy.
Before we discovered calorific rays (infrared) or chemical rays (ultraviolet), we were playing with the visible spectrum. Spectroscopy allowed us to divide up light into its different wavelengths and observe the universe. You can tell a lot about the composition of something by the frequency and amplitude of the light it emits.
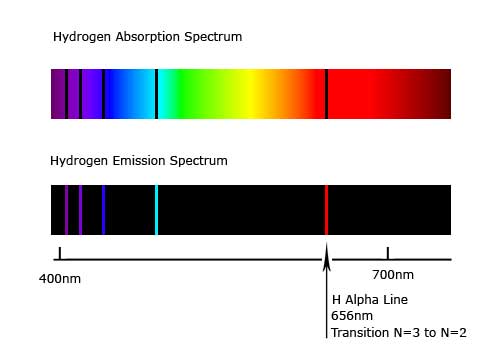
Early scientists (like Herschel), noticed that certain spectra had dark lines. These are absorption lines, when atoms (like hydrogen) absorb certain light frequency.
Johann Balmer noticed that hydrogen absorbed certain frequencies. This helped us discover just how much hydrogen was out there, and also determine the nature of the hydrogen atom itself. (Hardcore: the absorption lines happen when a hydrogen electron absorbs that photon's energy, thus causing it to jump or transition to the next electron shell.)
All elements exhibit absorption lines. While some are seen only "off-stage" (ie, in the infrared or ultrviolet or beyond, especially if red-shifted), many can be seen in the visible spectrum.
Here's the visible spectrum of the Sun. As you can see, there's plenty of dirty metals lurking within our nearest and dearest star. (Can you find sodium? Hint: it's orange.)
| Click here to embiggen. |
__________________________
Her Grace is best viewed in the visible spectrum.

3 comments:
Haha! I bet Her Grace is best viewed under ANY conditions. I can squint, and Her Grace still looks great in her profile pic.
I think I'm gonna have to study just to understand the entirety of this entry. But I'm working on it!
In my last MRI I looked absolutely fabulous!
Another informative post!
Post a Comment